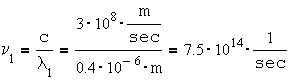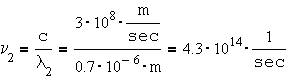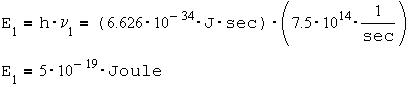|
The Interaction of Electromagnetic Radiation with Matter
(Emission and Absorption of Radiation) |
نعلم أن الذرة تكتسب طاقة وتفقدها بصورة مستمرة وإن انتقال الطاقة إلى الذرة يتم بواسطة طريقتين هما:
-
Collisions with other atoms, and the transfer of kinetic energy as a result of the collision. This kinetic energy is transferred into internal energy of the atom.
-
Absorption and emission of electromagnetic radiation
وحيث أن عملية الليزر تعتمد على انتقال الطاقة من خلال امتصاص Absorption الاشعاع الكهرومغناطيسي ثم تكبيره وانبعاثه emission على شكل شعاع ليزر، لذا سندرس ظاهرة الامتصاص والانبعاث.
The Interaction of Electromagnetic Radiation with Matter
The interactions between electromagnetic radiation and matter cause changes in the energy states of the electrons in matter.
Electrons can be transferred from one energy level to another, while absorbing or emitting a certain amount of energy. This amount of energy is equal to the energy difference between these two energy levels (E2-E1).
When this energy is absorbed or emitted in a form of electromagnetic radiation, the energy difference between these two energy levels (E2-E1) determines uniquely the frequency (ν) of the electromagnetic radiation:
(ΔE) = E2-E1 = hν
| Example
The visible spectrum wavelength range is: 0.4 – 0.7 [mm] (400-700 [nm]).
a) What is the frequency range of the visible spectrum. b) What is the amount of the photon’s energy associated with the violet light, compared to the photon energy of the red light.
Solution:
The frequency of red light:
The difference in frequencies:
The energy of a violet photon:
The energy of a red photon:
The difference in energies between the violet photon and the red photon is:
2.15*10-19 [J]
This example shows how much more energy the violet photon have compared to the red photon. |
Question:
Calculate in units of Nanometer, the wavelength of light emitted by the transition from energy level E3 to energy level E2 in which:
E1 = 0 [eV]
E2 = 1.1 [eV]
E3 = 3.5 [eV]
Emission and Absorption of Radiation
Every system in nature “prefers” to be in the lowest energy state. This state is called the Ground state.
When energy is applied to a system, The atoms in the material are excited, and raised to a higher energy level.
(The terms “excited atoms“, “excited states“, and “excited electrons” are used here with no distinction)
These electrons will remain in the excited state for a certain period of time, and then will return to lower energy states while emitting energy in the exact amount of the difference between the energy levels (DE).
If this energy is transmitted as electromagnetic energy, it is called photon.
The emission of the individual photon is random, being done individually by each excited atom, with no relation to photons emitted by other atoms.
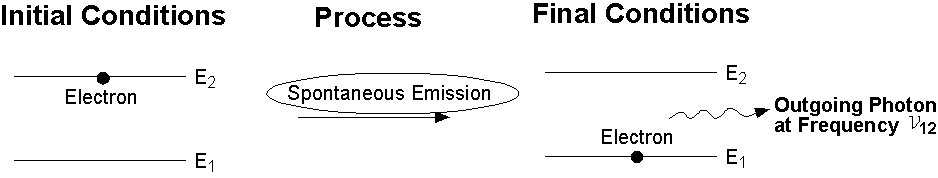
When photons are randomly emitted from different atoms at different times, the process is called Spontaneous Emission. Since this emission is independent of external influence, there is no preferred direction for different photons, and there is no phase relation between photons emitted by different atoms.
Spontaneous emission is one of a family of processes, called relaxation processes, by which the excited atoms return to equilibrium (ground state).
This “classic” explanation assumes that the specific frequencies emitted by an excited atom are the same as the characteristic frequencies of the atom, which means that the emission spectrum is identical to the absorption spectrum.
Possible Processes Between Photons and Atoms
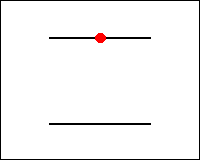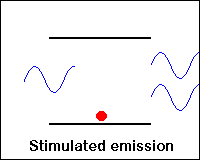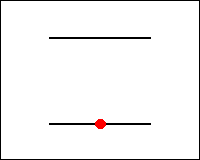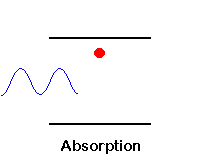
Three possible processes between photons and atoms: absorption, spontaneous emission, and stimulated emission.
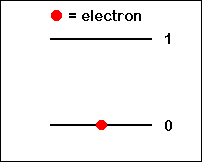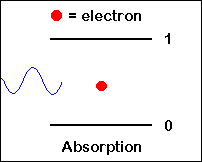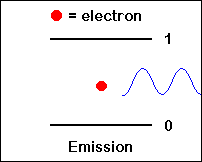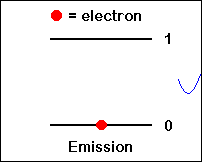
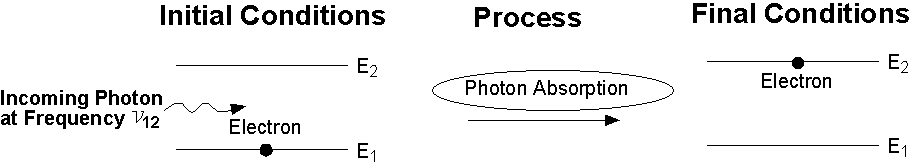
Photon Absorption: A photon with frequency n12 hits an atom at rest (left), and excites it to higher energy level (E2) while the photon is absorbed.
Spontaneous emission of a photon: An atom in an excited state (left) emits a photon with frequency n12 and goes to a lower energy level (E1).
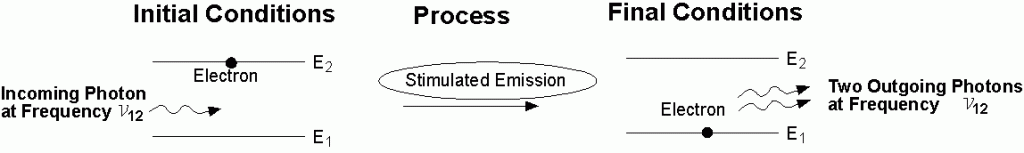
Stimulated emission of a photon: A photon with frequency n12 hit an excited atom (left), and cause emission of two photons with frequency n12while the atom goes to a lower energy level (E1).
| We saw that the process of photon absorption by the atom is a process of raising the atom (electron) from a lower energy level into a higher energy level (excited state), by an amount of energy which is equivalent to the energy of the absorbed photon. |
|
|
|
Absorption & Spontaneous emission |
|
|
Stimulated Emission |
Remember that two photons with the same wavelength (frequency) have the same energy:
E = hn = hc/λ
The incoming photon does not change at all as a result of the stimulated emission process.
As a result of the stimulated emission process, we have two identical photons created from one photon and one excited state. Thus we have amplification in the sense that the number of photons has increased.
This is the process that was explained in the introduction:
Light Amplification by Stimulated emission of Radiation = LASER
ِAverage Lifetime
Atoms stay in an excited level only for a short time (about 10-8[sec]), and then they return to a lower energy level by spontaneous emission.
Every energy level has a characteristic average lifetime, which is the the average time the electron exists in the excited excited state before making a spontaneous transition. Thus, this is the time in which the excited atoms returned to a lower energy level.
According to the quantum theory, the transition from one energy level to another is described by statistical probability.
The probability of transition from higher energy level to a lower one is inversely proportional to the lifetime of the higher energy level.
In reality, the probability for different transitions is a characteristic of each transition, according to selection rules.
When the transition probability is low for a specific transition, the lifetime of this energy level is longer (about 10-3 [sec]), and this level becomes a “meta-stable“ level. In this meta-stable level a large population of atoms can assembled. As we shall see, this level can be a candidate for lasing process.
When the population number of a higher energy level is bigger than the population number of a lower energy level, a condition of “population inversion“ is established.
If a population inversion exists between two energy levels, the probability is high that an incoming photon will stimulate an excited atom to return to a lower state, while emitting another photon of light. The probability for this process depend on the match between the energy of the incoming photon and the energy difference between these two levels.